Standardization is the activity of establishing rules and characteristics for the purpose of their mandatory and voluntary reuse, aimed at achieving orderliness in the field of medical care. Based on the legislation to documents in the field of healthcare standardization …
The history of standardization in domestic healthcare
Standardization is the activity of establishing rules and characteristics for the purpose of their mandatory and voluntary reuse, aimed at achieving orderliness in the field of medical care. Based on the legislation, documents in the field of healthcare standardization include procedures for the provision of medical care, provisions on the organization of the provision of medical care, standards of medical care, clinical recommendations (treatment protocols), national standards in the field of medical care, industry standards, and management standards. , logistics and information support.
Scientists, practitioners, and experts are making ever-increasing demands on the current healthcare standardization system, including those related to the unresolved issues of mandatory and voluntary application of medical care standards, their role in assessing the quality and accessibility of medical care, etc. Forming proposals on these issues is impossible without understanding ways of becoming standardization in Russia, which step by step, starting from the end of the nineteenth century. to this day is presented in this article. Four main stages of this path have been identified: 1992–2001. – creation of unified state standards for the quality of medical care; 2002–2006 – formation of national standards and standards of organizations; 2007–2011 – standards of medical care and medico-economic standards; 2012 to the present – federal procedures for the provision of medical care and standards of medical care. In conclusion, the article lists the legal problems of applying the standards.
The main stages in the development of standardization of medical care
1 For example: “Instruction on the signs of true and imaginary death”, “Instruction for doctors during judicial examination and autopsy of dead bodies”, “Rules on how to proceed when examining dead bodies, when there is a suspicion of poisoning”.
The next stage in the development of standardization was the transitional period of the Russian economy in the 1990s. XX century and the publication of the Law of the Russian Federation of June 10, 1993 No. 5154-1 "On Standardization". From 1991 to 2007, there was a rule on accreditation of medical institutions by determining their compliance with established professional standards, introduced by the Law of the RSFSR of June 28, 1991 No. 1499-1 “On the medical insurance of citizens in the RSFSR”. Order of the Ministry of Health of Russia dated June 28, 1993 No.148 "On Licensing and Accreditation of Medical Institutions" regulated the procedure for creating licensing and accreditation commissions under the territorial health authorities. The Central Republican Commission for Licensing and Accreditation of the Ministry of Health of Russia and the Commission for Licensing and Accreditation of Medical Institutions of Republican Subordination were established.
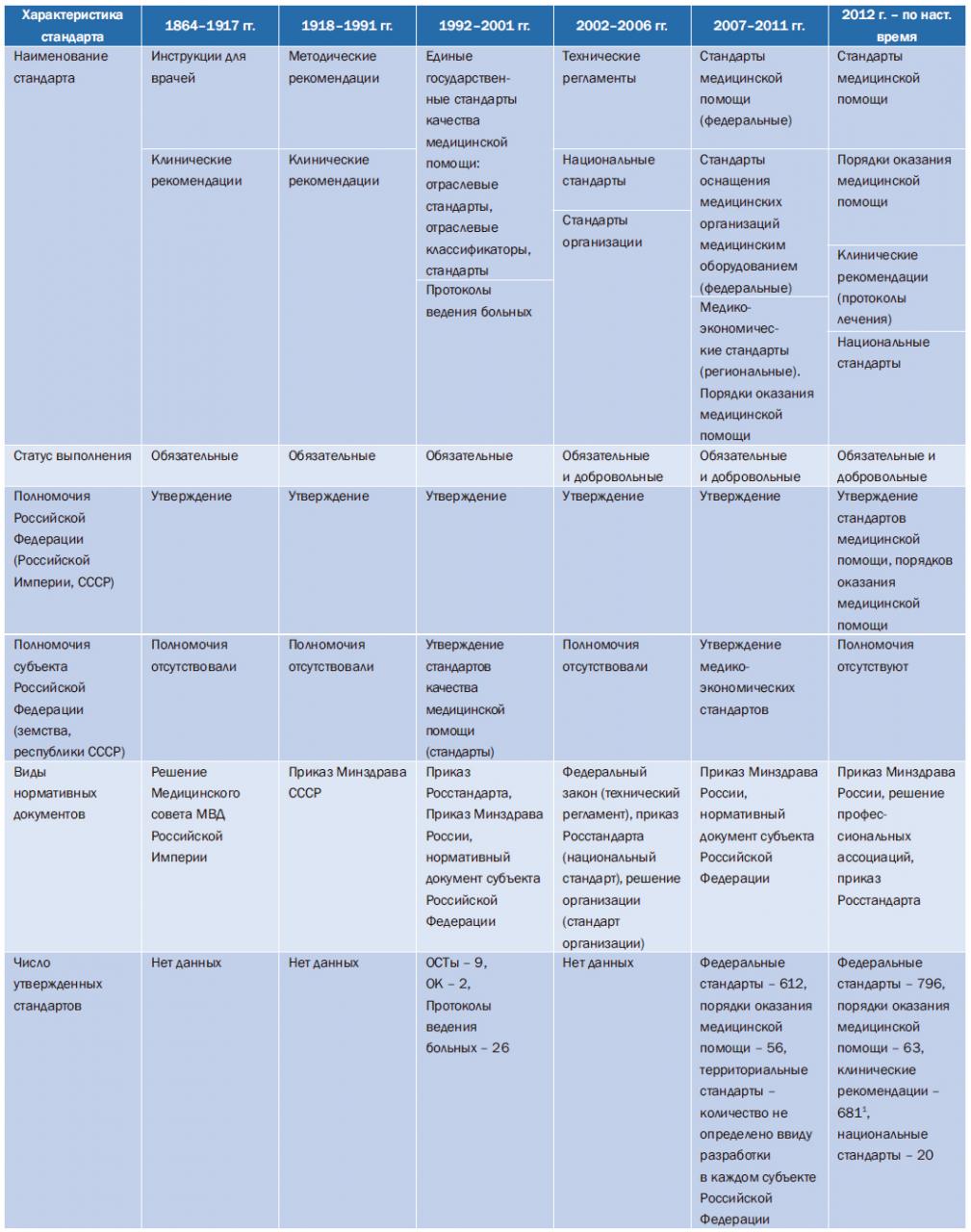
In accordance with the order of the Ministry of Health of Russia No. 363 and the MHIF No. 77 of October 24, 1996 "On improving the quality control of medical care for the population of the Russian Federation", licensing and accreditation commissions were determined as subjects of non-departmental quality control of medical care. These commissions were created in all subjects of the Russian Federation. The Ministry of Health of Russia issued normative acts regulating the accreditation of structural units of medical institutions. The main stages in the development of standardization of medical care are presented in Table. one.
Figure 1. Stages of development of standardization in healthcare in Russia
Uniform state standards for the quality of medical care (1992–2001)
In pursuance of this Decree, Order No. 277 of the Ministry of Health of Russia dated October 16, 1992 “On the Creation of a System of Medical Standards (Regulations) for Providing Medical Care to the Population of the Russian Federation” approved the Temporary Regulations on Medical Standards (Regulations) in the Medical Insurance System, by which medical standards classified depending on the administrative-territorial division (state, territorial) and according to the professional principle (diagnostic, medical and technological, treatment quality standards, medical and economic, medical and technological, scientific and medical standards, professional standards), as well as international standards (WHO). To develop and coordinate the application of medical standards, an appropriate department for medical and economic standards was created under the Ministry of Health of Russia.
"Basics of the legislation of the Russian Federation on the protection of the health of citizens" dated July 22, 1993 No. 5487-1 (in the original version – in paragraphs 11 and 15 of article 5) ensuring a unified technical policy in the field of the pharmaceutical and medical industry, approval of state standards of the Russian Federation , specifications for medical products, the establishment of quality standards for medical care and control over their observance are the responsibility of the Russian Federation. The procedure for providing primary health care (PHC) is established by the governing bodies of the municipal health care system on the basis of regulations of the Ministry of Health of the Russian Federation, the State Committee for Sanitary and Epidemiological Surveillance of the Russian Federation, ministries of health of the republics within the Russian Federation, legal acts of the autonomous region, autonomous districts, territories, regions , the cities of Moscow and St. Petersburg (Article 38).First medical care is in the manner prescribed by the Ministry of Health of the Russian Federation (Article 39).
State medical standards were developed by independent experts on the instructions of the Ministry of Health of Russia and were approved by the relevant expert commissions. All standards were developed by diseases (based on the nosological principle) in accordance with the international classification of diseases (hereinafter – the ICD) and the international classification of procedures in medicine.
Based on federal state standards of medical care to the health authorities of the subjects of the Russian Federation, territorial medical standards were developed, which determined pricing in the system of compulsory health insurance (OMS) for diagnostic and medical and technological standards. In order to unify approaches to postponic assistance for a treated patient (finished treatment), health and economic standards (MES) were developed by healthcare authorities (MES) for which tariffs for paying for medical care provided to a patient with a certain disease were established.
This made it possible to be introduced since 1993 within the framework of the OMS payment of the hospital services for the completed case of hospitalization on tariffs, differentiated in accordance with the classification adopted in the subject's subject (clinical-statistical groups, medical and economic standards, etc.).
The implementation of payment of stationary medical care for the completed case allowed, on the one hand, to reduce costs, motivate medical staff to improve labor productivity and KMP, and on the other hand, it led to certain difficulties in mutual settlements between the regions for the medical assistance rendered to specific citizens, since tariffs were calculated individually For each medical institution or types of institutions.
In order to ensure the conceptual unity and coordination of work on the establishment of regulatory documents on standardization in health care, harmonization with international systems for standardization of the Collegium of the Ministry of Health of Russia, the State Committee for Standardization, Metrology and Certification and the Council of Executive Directors of Territorial Funds of the OMS adopted a joint decision of December 3, 1997 . "On the basic provisions of standardization in health care." Also on July 21, 1998, the Ministry of Health of Russia, the Federal Fund of the OMS and the State Standard of Russia, the program of work on the creation and development of a standardization system in health care, which became the basis of the standardization system during this period. Since the end of 1999, work has been actively conducted on the creation of the most important documents of the standardization system – the protocols of patient management, including a detailed description of the technologies and procedures for the diagnosis and treatment of diseases.
By the end of 200626 protocols were approved. The methodological basis for the creation of these documents was determined by the OST 91500.09.0001-1999 "Protocols for the conduct of patients. General requirements". In 1998-2001. Fundamental sectoral standards have been developed. For example, the accepted OST 91500.01.0007-2001 "Standardization system in health care. The main provisions "established general organizational and technical rules for conducting work on standardization, form and methods of interaction between the subjects of the health care industry when creating and applying standardization regulatory documentation.
Industry classifiers were adopted, determining the classification of medical services and employees of the basis for payment for medical care: OK 91500.09.0001-2001 "Simple medical services", OK 91500.09.0002-2001 "Sophisticated and comprehensive medical services", OST 91500.09.0003-2001 Complex and complex medical services. Compound".
National Standards and Standards of Organizations (2002-2006)
In accordance with the indicated law, standardization is activities to establish rules and characteristics for their voluntary multiple use, aimed at achieving orderliness in the areas of production and product circulation and improving the competitiveness of products, works and services. Standardization is carried out mainly in order to increase the level of safety of the life or health of citizens, the property of individuals or legal entities, state or municipal property, environmental safety, security of life or animal and plant health.
The provisions of this law, extending its effect on medical care, were a step back in the development of standardization as a basis for quality management of medical care.
It should be noted that the quality of medical care is determined by such criteria as efficiency, safety, accessibility, timeliness, effectiveness, and the Federal Law "On Technical Regulation" mainly regulated security issues and did not actually ensure the regulation of the basic components of the CMP.
The conflict between the norms of the Law of July 22, 1993 No. 5487-1 "The Fundamentals of the Legislation of the Russian Federation on the Protection of Citizens" (hereinafter referred to as the foundations) and the Federal Law "On Technical Regulation" was allowed in favor of the latter, since on these issues he He was a special law, and the foundations are a general law.
At the same time, since 2005, within the framework of the Federal Law "On Technical Regulation", the established technical committee on the standardization of "Medical Technologies" was carried out work on the development of national standards in the field of health.
Medical Care Standards and Medical Economic Standards (2007-2011)
Thus, it was possible to establish federal standards of medical care as "minimal" and medical care standards, which should have been federal or higher.Also this law introduced changes in Art. 38 and 39 Basis, concretizing powers to establish the procedures for providing primary health care and emergency medical care.
Thus, the procedure for the provision of primary health care is established by legislation in the field of the protection of citizens' health, the SMP – the federal executive authority, carrying out regulatory and legal regulation in the health sector. Based on these rules, as well as established by the Decree of the Government of the Russian Federation of June 30, 2004 No. 321 "On approval of the Regulation on the Ministry of Health and Social Development of the Russian Federation", the powers of the Ministry of Health and Social Development of Russia approved the relevant procedures for medical care. In order to develop standardization in health care by the Federal Law of January 29, 2006 No. 258-FZ "On Amendments to Selected Legislative Acts of the Russian Federation in connection with the improvement of the delimitation of powers", paragraph 1 and 3 of Art. 6 Amended in Art. 5 The foundations of the powers of the federal state authorities in the field of citizens' health, which are emphasized from January 1, 2008 to the Ministry of Health and Social Development of Russia to establish medical care standards, standards for equipping healthcare organizations with medical equipment and other federal healthcare standards.
Also paragraph 8 of Art. 6 of this Law since January 1, 2007 Section VIII of the foundations supplemented with Article 37.1. Types of Medical Aid ", in which the procedures for the provision of PMSP, SMP and specialized, including high-tech, medical care are established by the federal executive body, carrying out functions to develop public policies and regulatory legal regulation in the health sector.
After consolidating in June 2008, the powers to apply the procedure for the provision of specialized medical care for the Ministry of Health and Social Development of Russia began work on the development of the procedure for the provision of specialized medical care, which ended in April 2010 to the powers of the state authorities of the constituent entities of the Russian Federation in the field of citizens' health assignment – Economic standards (MES) in accordance with federal standards of medical care. This allowed to combine medical care standards and MES in homogeneous groups based on close cost and uniform medical technologies and further form tariffs taking into account regional differences in the level of wages, drug prices and medical products
It even more strengthened the legal conflict in the field of standardization regulation in health care. In this regard, the expert community has insisted that the standardization of medical care is derived from under technical regulation legislation. Federal Law of May 1, 2007No. 65-FZ "On Amendments to the Law" On Technical Regulation "" found that its effect does not apply to relations related to the application of medical care measures (except for the development, adoption, application and execution of mandatory product requirements, Including drugs, medical equipment, food products).
By this time, 612 standards were approved by the orders of the Ministry of Health and Social Development of Russia. Orders had a recommendatory nature and in the Ministry of Justice of Russia were not registered. At the same time, in the process of developing standards, there was no publicity, the developers did not represent the interests of interdisciplinary groups, the conflict of interest was not taken into account, the results of public discussion were not published.
Since the end of 2007, the development and approval of the standards of medical care for the Ministry of Health and Social Development of Russia is suspended due to the lack of relevant authority. The Federal Agency for Technical Regulation and Metrology, endowed with the State Function for the Organization of Expertise Projects of National Standards and their Approval, on the basis of the appeals of the Ministry of Health and Social Development of Russia canceled from January 1, 2010 all approved earlier as the GOST reports of patients, including GOST R 52600.0-2006 "Protocols for the maintenance of patients. General. "
Order of the Government of the Russian Federation of February 28, 2006 No. 266-p Approved the Development Concept of the National Standardization System, which includes provisions on the problems of the development of the national standardization system in Russia until 2010, containing goals, objectives and directions for the development of the national standardization system.
In the period 2005-2007 In order to improve the further development of certain types of medical care, orders of the authorized federal executive body in the field of health, the provisions were approved on the organization of the activities of a medical organization, a structural unit, a specialist doctor, as well as approximate (recommended) lists of medical equipment (equipment table) in order to Medical implementation.
An important milestone in the development of standardization was the order of the Ministry of Health and Social Development of Russia of August 11, 2008 No. 410N "On the Organization in the Ministry of Health and Social Development of the Russian Federation, work on the development of the procedures for the provision of individual species (by profiles) of medical care and medical care standards" – approved the Regulation on Organization of work on the development of the procedures for the provision of individual species (by profiles) of medical care and standards of medical care.
In the period 2009-2010 Approved 28 orders of medical care for disease profiles. The order has failed in 2011, but the other procedure of the Ministry of Health of Russia has not yet been determined.
Thus, the developed, approved and currently applied standards of medical care are developed without proper discussion according to a procedure that has not yet been formalized. Also, the registration of new medical technologies in the period from 2007 to 2011, including new methods of prevention and diagnostics, according to which the issuance of permits for their use is attributed to the powers of federal government authorities, can also be attributed to the standardization of processes in healthcare.
According to the order of the Ministry of Health and Social Development of Russia dated July 20, 2007 No. 488 “On Approval of the Administrative Regulations of the Federal Service for Supervision of Health and Social Development for the Performance of the State Function of Issuing Permits for the Use of New Medical Technologies”, new medical technologies included those proposed for the first time for use on territory of the Russian Federation or improved sets of methods (techniques, methods) of treatment, diagnosis, prevention, rehabilitation, means by which these methods were carried out.
As of November 30, 2011 24091 new medical technologies were registered. This was the final year of medical technology registration. From January 1, 2012 after the entry into force of Article 14 of the Federal Law of November 21, 2011. 323-FZ "On the Fundamentals of Protecting the Health of Citizens in the Russian Federation", the registration of medical technologies has been suspended, the authority of the federal public authorities in the field of healthcare to issue permits for the use of new medical technologies has been excluded, as well as the requirement to use only preventive methods in healthcare practice , diagnostics, treatment, medical technologies permitted for use in the prescribed manner.
Thus, as of January 1, 2012, the function of issuing permits for the use of new medical technologies has been completely terminated, and therefore no permit is required to apply new medical technologies.
Separately, it is necessary to dwell on the conflict that arose again in 2011 between the norms of the Basic Legislation of the Russian Federation on the protection of the health of citizens and the Federal Law "On Technical Regulation". As noted above, the Federal Law of May 1, 2007 No. 65-FZ “On Amending the Federal Law “On Technical Regulation”” amended paragraph 4 of Art. 1 of the Law on Technical Regulation, within the framework of which medical care was withdrawn from the scope of this Federal Law, with the exception of cases of development, adoption, application and implementation of mandatory requirements for products, including medicines, medical equipment, food products. The same law approved a new version of Art. 11 of the Law on Technical Regulation, according to which the purpose of standardization is, among other things, to ensure the quality of services. In 2011 a change has been made (clause 4 of Art.1 of the Federal Law "On Technical Regulation"), which returned the norm on medical care under the law.
Thus, the standardization of quality assurance of medical services is carried out within the framework of the Law on Technical Regulation. It should be noted that according to the Federal Law of November 21, 2011 No. 323-FZ “On the Basics of Protecting the Health of Citizens in the Russian Federation”, the quality of medical care is a set of characteristics that reflect the timeliness of the provision of medical care, the correct choice of methods of prevention, diagnosis, treatment and rehabilitation in the provision of medical care, the degree of achievement of the planned result, and is determined by criteria such as efficiency, safety, accessibility, timeliness, and effectiveness.
The criteria for evaluating the CMP are formed by groups of diseases or conditions based on the relevant procedures for the provision of medical care, standards of medical care and clinical recommendations (treatment protocols) on the provision of medical care, developed and approved by medical professional non-profit organizations.
In order to implement the Law on Technical Regulation, the Technical Committee for Standardization "Medical Technologies" (hereinafter – TC 466), established in 2004, resumed its work. at the Federal Agency for Technical Regulation and Metrology. The purpose of organizing and carrying out work on standardization within the framework of TC 466 is to create a system of national standards recommended for practical use by medical organizations.
Yes, in 2014. Rosstandart approved GOST R 56034-2014 "Clinical recommendations (treatment protocols)" and GOST R 56044-2014 "Assessment of medical technologies" introduced by TK 466.
Thus, since 2011 and currently, within the framework of two federal laws, clinical recommendations (research protocols) are being developed and approved, which introduces uncertainty into healthcare practice. According to the authors, standardization in the healthcare sector should be carried out exclusively within the framework of a special law that regulates relations arising in the field of protecting the health of citizens in Russia. Therefore, it is necessary to return to the issue of clarifying the legislation, excluding from the scope of the Law on Technical Regulation the relations arising in the provision of medical care.
Federal procedures for the provision of medical care and standards of medical care (2012 – 2015)
Among the priority areas in the field of national standardization are medical devices, medical technologies and pharmaceuticals, biotechnology, ensuring labor safety and maintaining health in terms of establishing technical requirements for products, as well as enterprise management, conformity assessment, consumer protection.The new stage in the development of the standardization system in health care is associated with adopted November 21, 2011. Federal Law No. 323-FZ "On the basis of the health of citizens' health in the Russian Federation" (hereinafter – the law), which basically entered into force on January 1, 2012.
Part 5 tbsp. The 32 law is assigned the obligation of the authorized federal authority to establish provisions on the organization of medical care for types, conditions and forms of providing such assistance.
However, in 2012 Approved the Regulation on the organization of the provision of the PMD adult population and only at the end of 2014. Approved the Regulation on the organization of the provision of specialized, including high-tech, medical care. Prior to that, the procedure for the organization of the provision of specialized assistance in the year of 2010 was operating.
The fundamental difference between the new provision in establishing the rules for organizing the provision of not only specialized, but also high-tech medical care. In the emergency, including emergency specialized, medical care and palliative medical care so far, the provision of the organization has not been approved, and these types of medical care are regulated by the procedures.
From January 1, 2013 he entered into force part 1 Art. 37 of the law enshrining the norm on the organization and provision of medical care in accordance with the procedures for medical care, binding on the territory of Russia by all medical organizations, as well as in accordance or on the basis of medical care standards approved by the Ministry of Health of Russia and registered in the Ministry of Justice of Russia.
- application of the procedures for providing medical care and medical care standards in order to ensure the availability and quality of medical care (Article 10 of the Law);
- organization and provision of medical care in accordance with the procedures for providing medical care, as well as on the basis of medical care standards (Article 37 of the Law);
- organization and implementation of medical organizations (MO) of medical activities in accordance with the procedures for providing medical care and based on medical care standards (Article 79 of the Law);
- Formation of the program of state guarantees of free provision of medical care (GPG) and territorial programs for state guarantees of free provision of medical care to citizens (TPHH), taking into account the procedures for medical care and based on medical care standards (Article 80, 81 of the Law);
- the inclusion of information on the applied medical care standards into the personified accounting system (Article 94 of the Law);
- implementation of state control of quality and security of medical activities by conducting inspections by medical organizations with medical institutions of medical care and medical care standards (Article 88 of the Law);
- Participation of professional non-profit organizations in addressing issues related to violations of medical care and medical care standards (Article 76 of the Law).
Thus, the procedures for providing medical care and medical care standards determine the quality of medical care itself and the safety of medical activities.
According to the decree of the Government of the Russian Federation of August 13, 1997 No. 1009 "On approval of the rules for the preparation of regulatory legal acts of the federal executive authorities and their state registration" (hereinafter referred to as the Rules), the regulatory legal acts of federal executive bodies are issued only in the form of orders, orders, orders , Rules, instructions and regulations.
The regulatory legal act is a written official document adopted (published) in a certain form of a law-standing body within its competence and aimed at establishing, changing or canceling legal norms. In turn, under the legal norm, it is customary to understand the general obligatory state prescription of a permanent or temporary nature, calculated for repeated use. Acts prepared by federal executive bodies and registered in the Ministry of Justice of the Russian Federation are regulatory legal acts.
Medical care standards are approved by an authorized federal executive body (part 2 of Art. 37 of the Law) – the Ministry of Health of Russia. Orders on the approval of the standards of medical care are undergoing state registration in the Ministry of Justice of Russia, are subject to official publication within 10 days after their registration and are considered to be attached by virtue of their official publication.
The approval of the standard by order of the Ministry of Health of Russia, registered Ministry of Justice of Russia and officially published, makes it the norm of law. Medical care standards are a formalized description (in tabular form) of the amount of medical care, which should be ensured by a patient with a specific disease (nosological form), syndrome or in a specific clinical situation.
Standards are determined by types of medical care (IPP, SMP, specialized medical care and palliative medical care) and diseases. The standard indicates the form (emergency, urgent, planned) and the conditions for the provision of medical care (stationary, in the conditions of the day hospital, outpatient, outside the medical institution), a list of medical services, the frequency of the provision of the service and the average number of services (manipulations, appointments) for 1 The patient, a list of therapeutic drugs (LP), indicating the dosage and averaged application indicator, as well as the treatment time.
The requirements established by standards are designed for repeated use, they act regardless of whether specific legal relations arose or ceased.In this regard, failure to meet the standards of medical care, according to experts, can serve as a criterion for making a legal decision on the onset of legal liability for doctors. In particular, poor-quality medical care and poor treatment outcomes can be seen as a consequence of the failure to meet standards of medical care.
At the same time, with regard to the mandatory application of standards, the practice of implementing the Law revealed a number of contradictions. So, part 4 of Art. 37 of the Law establishes that the standard of medical care is developed in accordance with the nomenclature of medical services and includes average indicators: the frequency of provision and frequency of use of medical services; drugs registered in Russia with indication of average doses; medical devices implanted in the human body; blood components; types of therapeutic nutrition, including specialized therapeutic nutrition products, as well as other based on the characteristics of the disease (condition). At the same time, Part 5 of Art. 37 of the Law provides for the possibility of prescribing and using medicines, medical devices and specialized health food products that are not established by the standards of medical care, subject to the availability of medical indications, taking into account the individual characteristics of the patient, by decision of the medical commission.
The standards do not cancel the individual approach to the treatment of the patient. That is why Art. 73 "Obligations of medical workers and pharmaceutical workers" did not introduce a requirement for them to comply with the standards of medical care. According to this article, "… medical workers are obliged to provide medical care in accordance with their qualifications, job descriptions, official and official duties …" and carry out activities guided by the principles of medical ethics and deontology.
Thus, the concept of Part 5 of Art. 37 of the Law, laid down by the legislator, is not aimed at the possibility of using the standard of medical care as a binding document and used as an algorithm for treating a specific disease (condition) in a specific patient in a specific medical organization. This, in turn, is contrary to the provisions of Art. 79 of the Law, which provides for the obligation of medical organizations to carry out medical activities in accordance with the standards of medical care, as well as parts 2 and 3 of Art. 80 of the Law, the provisions of which establish that the provision of citizens with medicines, medical devices, specialized medical nutrition products is carried out in accordance with the standards of medical care within the framework of the SGBP and Part 1 of Art. 37 of the Law, which establishes the norm on the obligatory organization of medical care in accordance with the procedures and on the basis of standards of medical care.
It should be noted that inspections by the Prosecutor General's Office of the Russian Federation of compliance with legislation in the field of healthcare and on complaints from citizens about the availability and ILC are carried out on the basis of the obligatory observance of the standard of medical care. Failure to comply with the standard of medical care becomes the reason for the criminal prosecution of a medical worker.
Roszdravnadzor also carries out inspections based on the obligatory observance of the procedures for the provision of medical care and standards of medical care. Thus, the provisions of the Law on the mandatory standards of medical care are ambiguous and require significant clarification.
According to legal terminology, the wording "in accordance" means following some mandatory norm, rule, order, etc. According to the wording given in the Modern Explanatory Dictionary of the Russian Language by T.F. Efremova (2000), “in accordance” indicates the grounds for the action, which is the determining factor for the action. This formulation sets a strict vector of following or performing certain actions. The wording “based on” provides some direction to guide decision making.
To resolve the issues of correct interpretation of the norms of the law in 2013, amendments were made to Art. 79 of the Law, in which the organization and implementation of medical activities become the responsibility of a medical organization not “in accordance with” the standards of medical care, but “on the basis of”. At the same time, according to the authors, the vagueness of the legal structure of the Law remains, due to the presence of two substitute wordings: “in accordance with” and “based on” the standards of medical care.
Also, the legislator in Art. 88 of the Law, in terms of conducting inspections of medical organizations, changed the wording “observance” of the procedures for the provision of medical care and standards of medical care, meaning their exact (strict) execution, to a more loyal one – “application”. At the same time, in paragraph 1, part 3, Art. 80 of the Law, the provision on the provision of medical services, the appointment and use of medicines included in the list of Vital and Essential Drugs, medical devices, blood components, medical nutrition, including specialized medical nutrition products, for medical reasons in accordance with the standards of medical care in the provision of medical care in within the SGBP and TPGP.
According to part 7 of Art. 80 of the Law, the SGBP is formed taking into account the procedures for the provision of medical care and based on the standards of medical care, i.e. the discrepancy between the wordings “in accordance” (part 3 of article 80) and “based on” (part 7 of article 80) has not been eliminated, thus, the changes made do not answer the question about the role of standards in the system of medical care, despite their removal from Article 69, which defines the accreditation of health professionals. The innovation of the Federal Law of November 25, 2013 No.No. 317-FZ is an additional inclusion in Art. 64 clinical recommendations (along with the procedures for the provision of medical activities and standards of medical care) as the basis for the formation of cMP criteria.
Legal problems of application of standards
1. There is no procedure for the development, approval, application and revision of standards of medical care, as well as their structure. Although the legal status of the standards (orders of the Ministry of Health of Russia, registered by the Ministry of Justice of Russia) implies the obligation to publish and apply them in the prescribed manner, detailed explanations are required for what purposes, by whom and in what order these standards should be applied. In particular, the order of simultaneous use in the treatment of patients with several diseases is unclear, including in cases where prescriptions for different diseases may have an incompatible effect.
At the same time, there is no understanding of what degree of deviation from the standard as a binding document is allowed.
2. There is no list of standards of medical care that are mandatory for use. The principle by which the need to develop a standard for a particular disease is determined is not clear. According to experts, in accordance with the ICD, it is necessary to approve from 9 to 33 thousand standards of medical care, which must be constantly updated. This work requires large financial resources. As of the end of 2014, 63 procedures for the provision of medical care and 796 standards of medical care have been approved. At the same time, attempts were made at the legislative level to establish the administrative responsibility of a doctor for failure to comply with the standard of medical care. However, the legislator did not support such a provision.
3. The structure and content of the standards also raise a number of questions. The standards do not contain algorithms for treating a patient. It can be assumed that the standards should contain the most effective treatment technologies from both a clinical and an economic point of view, i.e. should contribute to ensuring the quality of medical care and improving the efficiency of healthcare. However, there is no information, as well as the relevant requirements, to what extent the latest treatment methods are taken into account in the development of standards, which allow improving clinical results and reducing the standard terms of treatment, the time spent by the patient in the hospital or even transfer part of medical technologies from the inpatient to the outpatient stage of medical care and thereby ensure more efficient use of health resources.
- on the part of doctors: the use by doctors of a simplified approach to treatment, the formal use of treatment technologies that are provided for in the standard, the refusal to use alternative or additional methods of treatment that are not indicated in the standards, which may be more effective for this patient;
- From the patients: the unreasonable requirement of drugs and procedures, which are provided in the standard, but in a particular case may not be shown by this patient leading to unjustified claims to doctors;
- from the auditory authorities: difficulties in determining the validity of the doctor's appointment (at the frequency of the <1, the criteria are currently not developed for which it is possible to judge whether the doctor's appointment is based, and, on the contrary, whether the deterioration / complication is connected with the fact that the doctor did not do this procedure);
- In terms of development and implementation into the practice of modern technologies: it is possible to slow down the process of introducing new technologies, since the procedure for developing and revising standards is complex and durable in time.
6. The contradiction of the law is that the standards of medical care are mandatory for execution by medical organizations and are optional to fulfill with medical workers. Of course, this situation requires discussion in the professional community and decision making.
All listed factors are constrained by the development of medical care management systems, the introduction of new medical care technologies, as well as ensuring the effectiveness of payment for medical care.
Information about authors
Nelly Borisovna Neligzina – Doctor of Medical Sciences, Professor (Moscow)
Filatov Vadim Borisovich – Doctor of Medical Sciences, Professor (Moscow) Borozdina Oksana Anatolyevna (Moscow)