ProSoft Information and technological support for 1C:Enterprise users (1C:ITS) is divided into two versions: PROF and TECHNO: Contract
ProSoft
Information technology support for 1C:Enterprise users (1C:ITS) is divided into two versions: PROF and TECHNO:
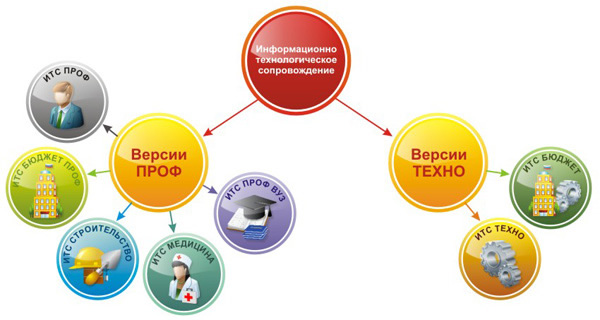
The 1C:ITS agreement for PROF versions provides users of 1C:Enterprise programs with the maximum amount of information and services provided by 1C and First Soft.
 Medicine is a very specific type of activity, which is largely regulated by law. General business information cannot fully meet the requirements of users of medical solutions.
Medicine is a very specific type of activity, which is largely regulated by law. General business information cannot fully meet the requirements of users of medical solutions.
Firm "1C" with the active participation of the company "RLS Patent" offers a variant of information technology support – ITS MEDICINE, which is focused specifically on the industry user.
The information system ITS MEDICINE is intended for managers, lawyers, accountants, pharmacists of medical institutions, IT-specialists in the use of "1C: Enterprise" programs in medical institutions.
The contract for ITS MEDICINE will be of particular interest to medical organizations with an in-hospital pharmacy, pharmacy chains and retail pharmacies, as well as other organizations involved in the circulation of medicines. ITS MEDICINE materials can be used as a data source for the Nomenclature reference book in configurations on the 1C:Enterprise 7.7 and 1C:Enterprise 8 platforms. Submit an application for ITS.
What will be interesting at ITS MEDICINE for a specialist of a medical organization:
- Head — normative documents regulating medical and pharmaceutical activities, other activities in the field of healthcare.
- Accountant of a self-supporting organization — a guide to business situations, unique guides on taxes and fees, assistance in reporting, advice on labor relations and personnel issues.
- Accountant of a public institution — articles by 1C methodologists and leading budget accounting consultants on the practical application of regulatory documents on budget accounting and its maintenance using 1C software products. Directory of financial and economic operations of a budgetary institution.
- to the pharmacist — a complete database of all medicines, dietary supplements and other pharmacy products registered in Russia; controlled prices for vital and essential medicines; contextual and exact search; color photographs of preparations
- IT specialist – updates of industry-specific, typical configurations for self-supporting and budgetary organizations, instructions for updating and methodological materials.
Review of new materials ITS MEDICINE October 2012
Register of Medicinal Products of Russia
In the September issue, the "Register of Medicinal Products of Russia®" was updated with an update as of September 18, 2012.
Added uploading information about drugs subject to withdrawal from the pharmacy and retail chains.
Materials of the section "Garant. Medicine"
The section "Garant. Medicine" includes the following materials of the information system "Garant-Medicine" version dated September 22, 2012:
- documents, acts of authorities that do not have an individual focus; valid at the time of transfer; extending their effect to the territory of the Russian Federation;
- forms of documents approved by state authorities related to medical topics, and forms developed by NPP "Garant-Service" together with the CPP (Legal Support Center).
The sample of documents includes laws, federal laws and the Fundamentals of Legislation, decrees of the Government of the Russian Federation, in one way or another related to the protection of the health of citizens and the provision of medical care to the population, the procedure for reporting and carrying out activities by medical institutions and pharmacy organizations, licensing activities in the field of healthcare, payment labor and certification of workers.
In addition to those listed, the sample includes documents from the following issuers: the USSR Ministry of Health, the Russian Ministry of Health, the RSFSR Ministry of Health, the USSR Ministry of Health and Medical Industry, the Russian Ministry of Health and Medical Industry, the Russian Ministry of Health and Social Development, the Federal Medical and Biological Agency of Russia, Roszdravnadzor, Rospotrebnadzor, Roszdrav, the Chief State Sanitary Doctor of the USSR, the Chief State Sanitary Doctor of the Russian Federation, the Chief State sanitary doctor of the FMBA of Russia, Standing Committee for Drug Control.
Solutions for healthcare organizations on the platform "1C:Enterprise 8"
The materials of the section "Methodological materials" have been updated:
- Web-SHMD
The section is aimed at developers of medical document templates for 1C:Medicine. Polyclinic and 1C:Medicine.Hospital products and developers of tools for integrating third-party medical information systems with 1C:Medicine products. The information in the section will be updated and updated in subsequent issues of ITS MEDICINE.
As of September 17, 2012, updates to the following solutions are included in the current release:

