Disseminated intravascular coagulation (DVS) is a primary or secondary violation of hemostasis, characterized by the association of phenomena of thrombosis and hemorrhage. DVS syndrome, which arose in the case of a primary violation, is always reversible when conducting successful therapy.
Diagnosis and treatment of disseminated intravascular coagulation syndrome (DVS)
Disseminated intravascular coagulation (DVS) is a primary or secondary violation of hemostasis, characterized by the association of phenomena of thrombosis and hemorrhage. DVS syndrome, which arose in the case of a primary violation, is always reversible when conducting successful therapy.
Hemostasis is the main physiological function that ensures the survival of the representatives of the mammalian class. Vascular integrity disorders lead to a solid reaction cascade, which determine the formation of stable clots and interrupt hemorrhages (Appendix 1). Many processes with an inflammatory component may cause excessive activation in the form of chain reactions and provoke multiple formation of thromboms. At the same time, excessive utilization of platelets and coagulation factors in the body leads to a gradual decrease in their quantity (deletion), which can be resolved by hemorrhagic diathesis. DVS syndrome is a complex pathological phenomenon manifested by clinical manifestations due to the association of contradictions arising at the level of hypercoagulation and hemorrhagic syndrome.
At the beginning of the article, the mechanisms of DVS development are considered, then attention is paid to treatment.
Appendix 1. Physiology of normal hemostasis
Understanding the peculiarities of the emergence, flow and treatment of DVS requires clear knowledge of the primary and secondary hemostasis, as well as on fibrinolysis processes and substances that limit excessive reactions (Fig. 1).
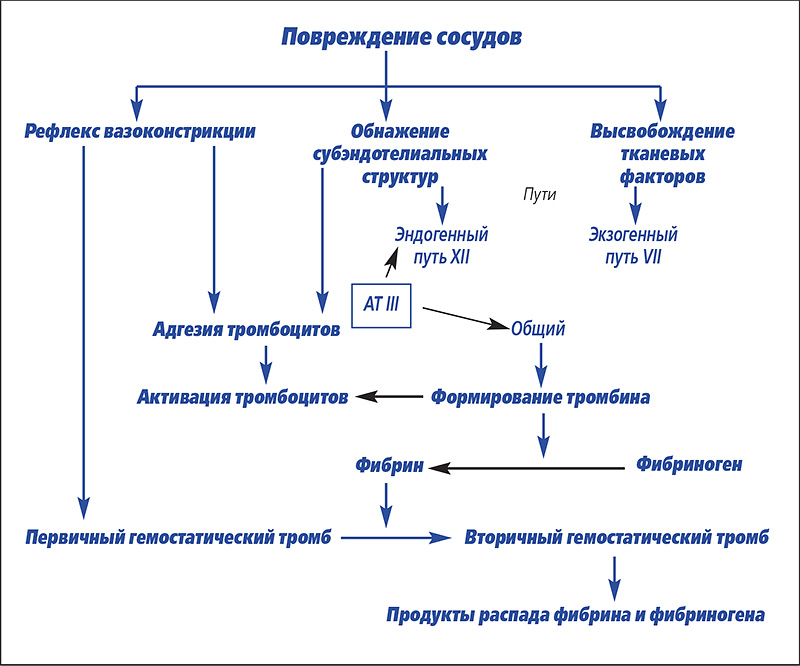 Picture 1. The overall view of the primary and secondary hemostasis phenomena (by Cloet-Chabre et Green).
Picture 1. The overall view of the primary and secondary hemostasis phenomena (by Cloet-Chabre et Green).
We recall the well-known hemostasis mechanisms, in addition to introducing the reader with some recent research in the field of physiology, with respect to local rheological * (* Riology (English. Rheology) – Area of physics, where we study the fluidity of matter and deformation of bodies as a result of the impact on them of various forces.) Control endothelium for hemostasis. In general, this single-layer epithelium in contact with circulating blood cells (platelets) secretes humoral factors whose action is based on ensuring the equilibrium of the vasomotor tone and blood flow (Moncada S., Palmer R.M.J., Higgs E.A., 1991).
Primary hemostasis
The primary hemostasis is manifested in the presence of a violation of the integrity of the vessel leading to the exposure of subendothelial collagen.There is a local vasoconstriction due to an imbalance between vasomotor tone and blood flow, which facilitates the adhesion of platelets to the damaged area. On the other hand, endothelial disorders lead to the disappearance of local antivasodilating and antiaggregating endothelial factors, which in turn enhances platelet aggregation. At the same time, the circulating Von Willebrand factor is activated. Activated platelets release many substances: adenosine diphosphate (ADP), thromboxane A2 and serotonin, which are responsible for vasoconstriction. On the other hand, they involve other platelets in this process, which form a kind of plug that forms a “white thrombus” from these cells. This "white thrombus" is unstable, its stabilization depends on the introduction of fibrin, the end product of plasma coagulation, which forms a protective network that adheres to fibrinogen receptors, α2 β3 integrin of activated platelets (Boudreux M.K., 1996). This synergy leads to the formation of a stable clot, which we call a "red clot".
Secondary hemostasis
Plasma coagulation includes endogenous and exogenous pathways in the process, which ultimately form fibrin. The endogenous pathway begins through the relationship between contact factors (factor XII, kallikrein, high molecular weight kininogen, HMWK) and subendothelial collagen. Through the direct activation of factor IX, an exogenous pathway is carried out. The exogenous pathway is activated when factor III (tissue factor or thromboplastin) comes into contact with blood components, providing a calcium-dependent complex with factor VII. Two pathways complete the activation of factor X, corresponding to the beginning of a common pathway with the final result of the transformation of prothrombin into thrombin. Thrombin catalyzes the formation of fibrin, starting from fibrinogen, and also causes platelet activation. Unlike conditions in vitro, differentiation of activation of endogenous and exogenous pathways in vivo is weakly expressed. Their activation begins simultaneously and factor IX of the endogenous pathway can be directly activated through the action of factor VIIa of the exogenous pathway. Activation in vivo two pathways of plasmatic coagulation at the same time can be supplemented with tissue factor.
Regulation of hemostasis
The regulation of plasma coagulation depends on the antithrombogenic quality of healthy endothelium located around the gap in the vessel, which limits the growth of a blood clot. Endothelial cells produce prostacyclin (PGI2), ADPase (destroys adenosine diphosphate), and nitric monoxide (NO). These substances are vasodilators and inhibitors of platelet aggregation (Hackner S.G., 1996). On the other hand, the endothelial surface contains glycosaminoglycans, as well as thrombin and thrombomodulin receptors (Welss D.J., Rachid J., 1998), which potentiate the activity of antithrombin III (AIII).Alpha-2 globulin, synthesized by the liver, is a very important inhibitor of plasma coagulation (80% anticoagulant activity) (Hackner S.G., 1996). Associating with its co-factor heparin, AIII forms inactive complexes with coagulation factors IIa, IXa, Xa, XIa and XIIa, which are then captured and eliminated by the liver. AIII deficiency can be caused by impaired synthesis in severe liver damage, loss in glomerulopathy (Ritt V.G., Rogers K.S., Thomas J.S., 1997) or exudative enteritis, or in the case of treatment with L-asparogenase. Deficiency of AIII is also stated in DIC due to its excessive consumption. Other less significant inhibitors of plasma coagulation are thrombomodulin and proteins C and S.
fibrinolysis
The fibrinolysis system is activated at the same time as the coagulation cascade and leads to the gradual dissolution of the fibrin clot. Fibrinolysis depends on the transformation of plasmogen into plasmin, which hydrolyzes fibrin into fragments: fibrin and fibrinogen degradation products (FDP). Fibrinolysis proceeds for several hours, ensuring complete dissolution of the thrombus and scarring of the damaged vessel.
ICE development
DIC is an acquired hemostasis disorder often encountered in veterinary medicine (Chabre B., Corlouer J.P.H., 1994). The disease is also referred to as "coagulopathy of consumption" and "disseminated intravascular thrombosis" (Feldman B.F., 1996), but this terminology is not currently universally accepted.
Nature
Many disorders of an inflammatory nature can provoke the development of DIC through various mechanisms that cause excessive formation of thrombin and fibrin in the hemocirculation system (Table 1). Leakage of tissue factors from damaged cells, endothelial damage with exposure of subendothelial structures, vascular stasis associated with metabolic acidosis and electrolyte disturbances are the main risk factors for DIC (Green R.A., 1995).
Table 1. Primary causes of DIC in dogs, cats and horses.
| Dog | Cat | Horse |
| Neoplasms – Hemangiosarcoma – Lymphoma/carcinoma |
Neoplasms – Lymphoma – Leukemia – Neoplasms of the mammary gland |
Neoplasms |
| infections – Bacterial nature: pyometra/bronchopneumonia, endotoxemia – Viral nature: plague – Parasitic nature: babesiosis (piroplasmosis) |
infections – Bacteria – Endotoxemia – Viruses: feline infectious peritonitis |
infections – Septicemia – Endotoxemia – Metritus – Pleuropneumonia — Viremiya |
| liver disease – hypovolemic shock – Anesthesia – volvulus of the stomach |
liver disease – Liver lipidosis |
Gastrointestinal disorder – Colic: – Hit – Enteritis / colitis – inversion |
| Tissue injury – Injury/burn – Heatstroke |
— | — |
| Other reasons – Complications during childbirth – Acute pancreatitis – Snake bite – Incompatibility at transfusion – Hemolytic anemia – Kidney amyloidosis |
Other reasons – Current of the intestine – acute pancreatitis – Lack of heart – Injury – intoxication – FIV / FELV (?) |
Other reasons – Liver disease – disease kidneys – Vasculit – Burn – Complications during childbirth |
The combination of three factors (hypercoagulation, damage to the vessels, vascular stas) is called "Virchova Tryiada". It makes up the general basis of all hypercoagulation syndromes (Hackner S.G., 1996). According to this principle of DVS, it can be a consequence of a set of primary disorders: neoplastic diseases, infection of viral, bacterial and parasitic nature, complications during childbirth, state of shock, injury, immune pathological processes and liver pathologies.
Mechanism
In order to have an idea of the development of DVS, three mechanisms should be taken into account.
- The most common cause of DVS is probably a violation of the exogenous path of plasma coagulation due to the release of a tissue factor from damaged or necrotic cells, for example, when the stomach breaks in the dog or the intestine at the horse.
- The second mechanism is due to the damage to the endothelial layer and the exposure of the subendothelial matrix, which activates the primary hemostasis and the endogenous path. We can observe this phenomenon during solar strike, vascular neoplasia (hemangiosarcoma) or during septicemia.
- Coagulation factors can be activated directly through excessive accumulation of enzymes in the hemocyerculation system after bite by some poisonous snakes or with acute pancreatitis.
The evolution of studies in this area made it possible to deepen knowledge about the launchers of the DVS development factors: excessive stimulation of immunity leads to general inflammation in the body (generalized inflammatory response syndrome), which underlies the activation of the coagulation system. Activated cytokines, such as tumor necrosis factor (Tumor Necrosis Factor, TNF), as well as interleukins are launchers. They are caused by macrophages to the examination of various factors of progulation, among which thromboplastin is the most important. On the other hand, the same cytokines stimulate the production of enzymes that induce inflammation, such as NO synthetase (NOS2) and cyclooxygenase (COX2). Unlike its homologues NOS1 and 3, COX1, they induce a thousandfold increase in humoral factors (NO, Prostaglandins) (TRONCY E., 1999), which, as a rule, has a favorable effect necessary to respond to the impact of an infectious agent. But in excessive amounts, these substances can provoke the development of the GGVR. Anti-inflammatory cytokines, in addition, lead to the expression of adhesions on the endothelium surface and cell circulating in cells, which in turn aggravates damage to the endothelial layer of vessels, exposing the subendothelial matrix and ensuring the re-emission of the tissue factor.The combination of these phenomena probably appears in almost all cases of DIC, in particular during sepsis (Bateman S.W., Mathewsky K.A., Abrams-Ogg A.C.G., 1998).
Effects
After being included in the blood coagulation cascade, excessive activation of platelets and coagulation factors ensures the sudden appearance of multiple thromboses. If the animal survived this phase, then a gradual decrease in the elements involved in hemostasis leads to the development of a hemorrhagic syndrome with anomalies of primary and secondary hemostasis. In circulating blood, we observe an increase in fibrinolytic activity and subsequent accumulation of fibrin and fibrinogen degradation products (FDP). At the same time, the absorption of natural regulators of hemostasis, in particular antithrombin III (AIII), can aggravate clinical symptoms.
Evolution
The manifestation of DIC depends on the nature, severity, course (superacute, acute or chronic), concomitant disease, the intensity of fibrin formation, AIII content, as well as the ability of the liver and bone marrow to compensate for blood clotting factors and absorbed platelets. Metabolic acidosis, hypoxia, vascular stasis and liver failure aggravate the clinical picture of the disease.
DIC becomes decompensated due to powerful stimuli, for example, during the development of acute ischemia, which causes an equivalent response in the system of inflammation and hemostasis. This sudden and rapid reaction does not allow compensatory mechanisms to be included in this process. Violations leading to thrombosis and hemorrhage become irreversible.
In chronic and compensatory DIC, a different situation is observed. The activation of the coagulation system proceeds imperceptibly and allows the body to find an equilibrium state due to an increase in the production of platelets and blood clotting factors. Clinical symptoms are absent or discrete. If DIC and primary disorders are not treated, then DIC, evolving into the stage of decompensation, manifests itself with the corresponding symptoms (Bateman S.W., Mathewsky K.A., Abrams-Ogg A.C.G., 1998).
Clinical picture
The clinician should consider examining a patient who is in the asymptomatic stage of DIC. At the same time, the clinical picture varies both in compensated and decompensated processes, depending on the course of the disease (chronic or acute). The clinical symptoms associated with DIC are sometimes difficult to observe in the chronic course of the disease. They can be detected in a general biochemical study. On the other hand, the symptoms associated with the primary disease may completely camouflage the signs of DIC (for example, in the case of hemoperitonitis, which can be caused by rupture of the spleen due to hemangiosarcoma or autoimmune anemia).
With the development of acute or hyperacute DIC in an animal, clinical signs of thrombosis, hemorrhage and / or shock are often noted.This encourages the search for a provocating factor or primary violation.
The symptoms observed during thromboembolism are usually associated with the functional insufficiency of the damaged organ. Obstruction at the microcirculation level or, which is less common, large-caliber vessels, can lead to acute renal failure, expressed by the disposage, ischemic necrosis of the organ in the abdominal cavity associated with Ileus. It is possible to violate circulation due to shock.
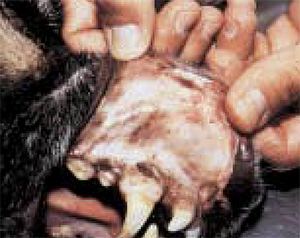
The horses of the economy often occurs during endotoxmia due to colic or septicemia. At the same time, clinical manifestation more often has a hemorrhagic, but thromboembolic nature (photo 1) (Morris D.D., 1998). Hemorrhages are manifested in all types of animals in the form of petechs (photo 2), bruises, ekkimosis, hematomas (photo 3) and bleeding arising at the level of mucous membranes or cavities, as well as melanomes and hematuria. In addition, we can observe long-term bleeding and hematoma during veins puncture or around the catheter introduced into it. In the postoperative period, in particular in the case of ischemia (stomach milling, the colic at the horse), the internal combustion engine can manifest itself in the form of bleeding from the wound for several hours or days after the operation.
The cat's cat is rarely observed and often proceeds. The chronic flow prevails (COUTO C.G., Hammer A., 1994).
 |
 |
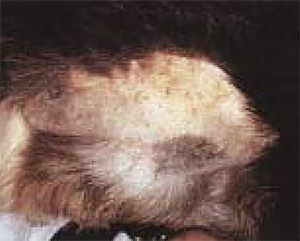 |
| Photo 1. Thrombosis of the jugular vein at the horse. | Photo 2. The presence of petechs at the level of the mucous membrane of the oral cavity in the sick dog. | Photo 3. The presence of echimosis on the surface of the skin. |
Diagnosis
DVS is characterized by a violation of the primary or secondary hemostasis with an increase in fibrinolysis and a decrease in the function of hemostasis regulators, in particular, at the level of antithrombin III. A biochemical study does not allow you to put the final diagnosis. Consequently, the clinical assumption on the economy is confirmed on the basis of a set of clinical symptoms and the results of a laboratory study (Table 2 and Appendix 2). Charges must be calculated, take into account the coagulation time (Temps de Quick., Cefalin's activity time) and determines fibrinogen concentrations, AIII and PDF. When bleeding, additional research is possible directly at the location of the patient. Counting shaped elements is often the first study method. Thrombocytopenia is observed in 80% of cases in DVS in the dog (Bateman S.W., Mathews K.A., Abrams-Ogg agg.g., 1998). At the beginning of the DVS development, thrombocytosis can be observed if the bone marrow exhibits a high degree of activity. Reducing the number of platelets often accompanies exacerbation of hypercoagulation. In case of urgent need, when there is no possibility to use the services of a laboratory, a microscopic study of blood smear under immersion (with an increase in lens × 100), it allows you to refine the number of platelets, provided that their normal content in the field of view of the microscope corresponds to 8-10 cells in the dog and 6-10 at the horse.In a cat, it is recommended to carefully examine a thin layer of blood smear applied to a glass slide in order to identify platelet aggregations.
Table 2. Biochemical assessment of hemostasis: practical recommendations
Appendix 2 When should you think about ICE?
A preliminary diagnosis of DIC is made on the basis of several clinical features. DIC may complicate the primary disorder or be only part of its overall manifestation. DIC is also included in the differential diagnosis of acquired hemorrhagic syndrome. Thus, we summarize the clinical situation, on the basis of which one should think about the presence of DIC.
DIC occurs when the function of primary and secondary hemostasis is altered.
If the animal has hemorrhages, the history and clinical examination often guide the diagnosis. It remains only to determine whether the violation is primary or not.
During the general spread of some neoplasms, in particular in the preoperative phase or prior to the administration of chemotherapy.
Hemangiosarcomas and malignant hemopathy are often complicated by DIC: early detection is essential before surgery or before prescribing L-Asparaginase.
In the process of spreading, a malignant formation leads to endotoxemia. It also occurs with disorders of the gastrointestinal tract of an infectious nature or may be associated with ischemia.
Gram-negative bacteria toxins provoke platelet hyperaggregation and can directly lead to plasma coagulation (Thomas J.S., Green R.A., 1998).
In the postoperative period, in particular when excising a neoplasm or due to a traumatic or ischemic phenomenon.
Directed clinical examination and proper biochemical analysis allow early detection of DIC. The prognosis varies depending on the reversibility of the primary disorder.
With a general systemic disorder
The development of the coagulation cascade can accompany many diseases. We are talking about careful monitoring in case of possible pancreatitis, hemolytic anemia, hyperthermia, bronchopneumonia, shock, colic, as well as infectious peritonitis in cats, hepatitis, etc. A partial examination is carried out by counting the quantitative content of platelets, the time of activated cephalin and temps de Quick. In the case of results indicating a violation, the next step is to identify the PDF and score AIII. The clinical assumption is the main one: normal parameters in the biochemical study cannot exclude the compensatory phase of DIC.
Bleeding may occur due to thrombocytopenia alone if the platelet count is below 30×109 per liter (Godeau B., Bierling P., 1997). Evaluation of plasma coagulation and platelet function is carried out in hemorrhagic syndrome associated with moderate thrombocytopenia.To do this, determine the time of bleeding from the mucous membrane of the vestibule of the oral cavity (in the cheeks), given that normally it should be less than five minutes.
With the exception of extensive hemorrhage or concomitant hemopathy, the number of red blood cells in DIC does not change significantly. Morphological examination of erythrocytes under a microscope is of great importance: schizocytes, keratocytes or helmet-shaped cells are often observed due to fragmentation of erythrocytes. This is explained by the fact that filaments of circulating fibrin adhere to erythrocytes and literally cut off part of their membrane during passage in the microcirculation system, thereby provoking a change in morphology. In any case, such anomalies and fragmentation of erythrocytes are not pathognomonic signs of DIC. They can be detected in anemia with Heinz bodies, as well as in anemia caused by iron deficiency and anomalies in the structures of the heart and blood vessels (for example, with hemangiosarcoma) (Slappendel R.J., 1988). In DIC, plasma coagulation is determined by temps de Quick (exogenous pathway), cephalin time with activator (endogenous pathway) and thrombin time (common pathway), which increased by more than 25% compared to control. This increase is secondary, associated with the absorption of serum coagulation factors and a decrease in their concentration (fibrinogen and factors V and VIII) (Green R.A., 1995). The activation time of cephalin increases to a greater extent than temps de Quick. However, sometimes the time of plasma coagulation is normal, in particular in the chronic course of DIC with compensatory production of coagulation factors by the liver. Fibrin and fibrinogen degradation products (FDP) are easily determined in the laboratory by the method of semi-quantitative agglutination, which is used in humanitarian medicine. Determination of PDF is relatively reliable in dogs and horses. There are difficulties in interpreting this indicator in a cat, as it can be unstable and varies (Slappendel R.J., 1988). A quantitative indicator in the range of 10-20 μg / ml is considered relatively specific (Dossin O., 1995; Furic F., Heripret D., Olivry T., 1992). An increase in PDF to 40 µg/ml in DIC is pathognomonic (Lassen E.D., Swardson C.J., 1995). Such an increase in the sudden appearance of fibrinolysis in differential diagnosis is classified as primary fibrinolysis (underdocumented disease in veterinary medicine) (Furic F., Heripret D., Olivry T., 1992; Green R.A., 1995). The phenomenon can also be with severe liver failure (impaired elimination of PDF) (Green R.A., 1995) or thromboembolism. Active fibrinolysis leads to hypofibrinogenemia, rarely seen in the horse, because fibrinogen is rapidly synthesized by the liver in response to an acute inflammatory response (Furic F., Heripret D., Olivry T., 1992; Lassen E.D., Swardson C.J., 1995). Total fibrinogen is most often reduced in the dog, but it has been shown that it can be within the physiological range in acute inflammatory or chronic disease, in which its production exceeds excess intake.Determination of the concentration of antithrombin III is very widely used to form a diagnosis of DVS and allows you to navigate in the treatment and forecast of the disease (Bateman S.W., Mathews K.A., Abrams-Ogg F.g.g., 1998; Green R.A., 1995). Antithrombin III forms an inactive complex with thrombin and coagulation factors, which are then eliminated by the liver. The reduced level of circulating AIII is a specific diagnostic element into DVS. More than 80% of susceptible diseases of dogs (Green R.A., 1995) and over 50% of horses (in the DVS study, associated with colic) AIII is significantly reduced (Welch R.D., Watkins J.P., Taylor T.S., COHEN N.D., Carter G.K., 1992). In a study published by Thomas J.S., Green R.A., 1998, all cats prone to DVS have a decrease in AIII. At the same time, the detection of AIII by more than 50% compared with the norm is a negative element in the disease forecast (Welch R.D., Watkins J.P., Taylor T.S., COHEN N.D., Carter G.K., 1992). Other most accurate methods for diagnosis are currently used in humanitarian medicine, but they are not worked out for pets. The determination of the amount of specific peptides of the pro -ugulating and fibrinolytic activation, as well as the consumption of inhibitors, can most early determine the presence of internal combustion in humans.
The diagnosis of DVS in veterinary medicine depends on the association of the component of the patient's clinical state (bleeding, related diseases predisposing to the engine) and the anomalies detected from its methods of laboratory research.
Thus, we believe that a three-time study indicating a violation of the number of platelets, coagulation time, PDF, as well as decreased AIII, fibrinogen and the change in the morphology of the erythrocytes is necessary for the diagnosis of DVS (Bateman S.W., Mathewc K.A., Abrams-Ogg agg.g., 1998 ) A set of laboratory research results allows DVS to differentiate from other coagulopaths (Table 3).
Table 3. Comparison of anomalies with an additional study in the case of internal combustion and hemostasis violations, often occurring in domestic animals.
PDF – fibrin degradation products and fibrinogen; + increase; – decrease, 1 – antithrombin III decreases with a pronounced liver disorder; 2 – Elimination of PDF may be late in with a pronounced liver disorders; 3 – Some erythrocyte anomalies can be detected during liver disorders in cellular cells.
| Thrombo cytopenia | Thrombo cytopathy | Antivitamin K. | Hemophilia | Failure liver |
DVS | |
| Count Thrombocyte |
— | norm | norm | norm | norm | — |
| Time bleeding from gums |
+ | + | norm | norm | norm | + |
| Time Activated Cefalin or Kaolina |
norm | norm | + 2 E. | + | + | — |
| Temps de Quick. | norm | norm | +1 E. | norm | + | + |
| Tombin time | norm | norm | + 3 E. | norm | + | + |
| Antithromlin III | norm | norm | norm | norm | — 1 | — |
| PDF. | norm | norm | norm | norm | norm or + 2 | + |
| Morphologists Erythrocyte |
norm | norm | norm | norm | Norma or Violations 3. |
Violations |
Treatment
DVS treatment needs to adjust the launchers. If this does not happen, despite the use of symptomatic therapy continues the activation of coagulation processes.Chronic and weakly heated internal combustion engine syndrome When liquidation of the root causes can be expected spontaneously. The purpose of the therapeutic intervention is to eliminate the causes of the disease, ensuring the normal functioning of AIII, braking the cascade of plasma coagulation and platelet aggregation, as well as in adapting supporting treatment to each case (Appendix 3). Sometimes it is difficult to apply fast and efficient treatment and eliminate the starting element, in particular with neoplasia or inflammatory process, for example, the infectious peritonite in the cat. According to the authors (Bateman S.W., Mathewc K.A., Abrams-Ogg agg.g.g., 1998; Doliger S., 1996; FURIC F., Heripret D., Olivry T., 1993) The neoplasms are one of the causes of the DVS development of the dog. Hemangiosarcomas, primary liver neoplasms or metastases, lymphoma, myeloma and carcinomas most often participate in this process (Doliger S., 1996). Detection of accompanying internal combustion engine exacerbate the disease forecast. Treatment of malignant neoplasms (surgery, chemotherapy, radiotherapy, etc.) should be carried out quickly after the start of the specific treatment of DVS.
Appendix 3. Algorithm for the treatment of DVS.
1. Eliminate factors for the development of the disease
The development factors of this pathology should be eliminated and are treated as quickly as possible, the effectiveness of treatment and determines the state of the FRO.
2. Maintain a state of normal hydration
It is necessary to intravenously enter the catheter and assign infusion therapy to adjust dehydration, restore the electrolyte and acid-alkaline balance, output the patient from the shock state.
3. Inhibit intravascular coagulation
* Heparin: 5-10 me / kg every eight hours.
100-200 me / kg every eight hours, if there is no improvement.
Efficiency is manifested by improving the overall state and normalization of coagulation time at a dose of heparin below 150 IU / kg of previously appointed.
* Purpose of blood:
One-piece blood is 10-20 ml / kg.
Fresh or fresh frozen plasma 6-10 ml / kg 1-2 times / day.
Hemotransphus is prescribed an animal as needed, but it should be borne in mind that it should contain AIII, also coagulation factors and sometimes platelets or red blood cells.
4. Supporting therapy and prevention of complications
Oxigenotherapy, antibiotic therapy, temperature control, hygiene, analgesia, monitoring of vital indicators (cardiovascular system, respiratory apparatus and kidneys).
DVS treatment arising from hypovolemic shock or septicemia may be crowned with success (Slappendel R.J., 1988) due to its reversibility after specific treatment (infusion therapy, antibiotic therapy, etc.). Supporting infusion therapy is the main element to ensure the survival of an animal exposed to the acute flow of DVS. Infusion isotonic solution allows you to restore the volume in the hemocirculation system, avoid microvaletration due to thrombing and venous state, adjust dehydration, metabolic acidosis, reduce the concentration of coagulation factors (Furic F., Heripret D., Olivry T., 1992).The purpose of infusions is based on determining the state of hydration, electrolyte composition and acid-base balance in the animal's body. If necessary, infusion solutions should be enriched with sodium bicarbonate and potassium chloride. The appointment of hypertonic or colloidal solutions (dextrans, hydroxyethylomedones) may be necessary to ensure hemodynamic parameters in the event that the patient is in a state of severe shock.
Oxygen therapy is prescribed when secondary respiratory disorders caused by pulmonary thromboembolism are detected in the animal, in case of a primary violation of the respiratory apparatus (purulent bronchopneumonia), as well as in severe anemia. Oxygen is supplied through a mask, a nasal probe, or by placing the animal in a sealed chamber.
Other methods of palliative treatment are also used, for example, antibiotic therapy for a bacterial infection, temperature control (heating or cooling), analgesia. Very close monitoring of the patient's condition is necessary, as it can deteriorate rapidly even if it appears to be stable. Evaluation of arterial and visceral venous pressure, as well as blood gases and urination (through a tube and a closed system of urination) are necessary elements that are used, despite the complexity in the organization in routine clinical practice.
One of the key points of specific treatment is the use of AIII. It is found in fresh blood, fresh or frozen plasma (prescribed at the rate of 10-20 ml/kg IV). When plasma is stored frozen, AIII remains stable for a year. Fresh blood is used for deficiency of erythrocytes and platelets.
Heparin is also used in the specific treatment of DIC. Several schemes for prescribing this drug have been proposed. The minimum therapeutic dose (5-10 IU / kg 3 times a day) and a low dose "low dose"(75-200 IU / kg 3 times a day) is used for mild symptoms of the disease. Medium (300-500 IU / kg three times a day) and high (750-1000 IU / kg three times a day) doses are used for severe thromboembolic or hemorrhagic manifestations. Heparin is predominantly administered subcutaneously or intravenously. It can also be injected into plasma 30 minutes before the start of transfusion, which ensures the formation of the active AIII-heparin complex under conditions in vitro. It completely loses activity if the threshold concentration of AIII in the animal is 40% below the physiological norm (Green R.A., 1995), as well as in the case of severe metabolic acidosis or inadequate tissue perfusion. Therapy with heparin is contraindicated for the period of the operation (it is preferable to inject blood or plasma until the end of the surgical intervention).
The effectiveness of treatment is always difficult to assess, because the normalization of coagulation time is masked by an increase in the concentration of prescribed heparin.Some authors recommend using low doses of the drug (75 IU / kg 3 times a day), which does not have a significant effect on the coagulation time, but at the same time it allows you to assess the improvement of the internal combustion system through the normalization of biochemical indicators (Slappendel R.J., 1988). On a person it was shown that the appointment of minimum therapeutic doses of heparin is also effective in the treatment of acute flow of DVS, as well as the appointment of higher doses.
Nonteroidal anti-inflammatory funds (NSAIDs) were proposed for the treatment of DVS in some animal species. The NSAIDs reduces platelet aggregation and product mediators (prostaglandins) products, and also cause analgesia. Flunixine Meglumine, Finaline (FluniXine Meglumine, Finaline) at the rate of 0.25 mg / kg can also be used for horses, for example during colic. For dogs, some authors offer the appointment of aspirin (5-10 mg / kg in the morning and in the evening) (Nelson R.W., Couto C.G., 1998). However, any special study in relation to the NSAID in order to identify their importance in the treatment of DVS in domestic animals did not spend. In this regard, they can only be used empirically and with caution, in view of their side effects (ulceration of the gastrointestinal tract and renal failure). Anti-inflammatory steroid drugs for the treatment of DVS are not recommended, because they reduce the activity of mononuclear phagocytes and, affecting catecholamines, potentiate vasoconstriction. Their use may occur if it is necessary to carry out specific therapy of primary disease (for example, autoimmune hemolytic anemia).
Conclusion
Knowledge in terms of DVS has recently evolved significantly, but the methods of diagnosis and treatment remained at the same level. Mortality is still high! This situation is explained by the severity of primary diseases and methods of diagnosis, which in veterinary medicine is departed (late consultation of patients, weak availability of laboratory research methods, etc.). In addition, there is no specific treatment of DVS. According to literature, the total mortality ranges within 50-78% (Bateman S.W., Mathews K.A., Abrams-OGG A.G.G., 1998), so the forecast is always discreet and dubious.
Currently, scientific research is underway against generalized inflammatory response syndrome. Perhaps diagnostic tests will be developed, providing early detection of hypercoagulation phenomena. The definition of fibronectin, D-dimer (fibrin degradation products) or activated platelets is currently inaccessible in routine clinical practice and borrowed from humanitarian medicine, or used under experimental studies. On the other hand, new treatment approaches may appear much faster. Three categories of drugs are already used in humanitarian medicine: endotoxins and mediators inhibitors responsible for inflammation; Inhibitors of coagulation and platelet system.These are Anti-TNF antibodies or antifactors of tissue nature, activated protein C or thrombomodulin, as well as platelet activating factor inhibitors (Wels D.J., Rachid J., 1998), may soon replenish part of our therapeutic arsenal in the treatment of SHVR and DIC.
Basic provisions
— Disseminated intravascular coagulation (DIC) is a complex pathological phenomenon that manifests itself clinically as a result of associations of contradictory states of increased coagulation and hemorrhagic syndrome.
– The clinical picture varies depending on the acute or chronic compensatory and decompensated course of the disease. In a cat, DIC is rare, and its clinical manifestations are often discrete, with a chronic course predominating (Couto C.G., Hammer A.S., 1994).
– Unfortunately, there is no biochemical study that allows you to make an accurate diagnosis. Therefore, the clinical suggestion of DIC is supported by the combination of symptoms and laboratory findings.
