Critical concentration of micelle formation The formation of micelles occurs when monomers bind to each other at concentrations exceeding a rather narrow range. Critical Concentration
Critical micelle concentration
The formation of micelles occurs when monomers bind to each other at concentrations exceeding a rather narrow range. The critical micelle concentration (CMC) is the concentration of a surfactant at which a large number of micelles appear in its solution, which are in thermodynamic equilibrium with molecules (ions). The formation of a G1AB micelle in a solution means the appearance of a new phase in it, which leads to a sharp change in any physicochemical property of the system.
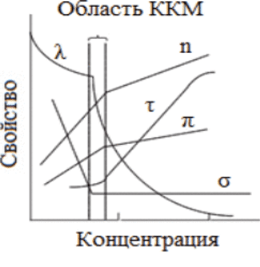
All methods for determining CMC are based on registering a break in a graph showing the concentration dependence of the physicochemical properties of surfactant solutions (surface tension σ, turbidity r, molar electrical conductivity R, osmotic pressure P, refractive index P (Fig. 5.3). One of the branches of such curves describes the properties of the system in the molecular state, the other in the colloidal state. The breaking point is considered corresponding to the transition of molecules into micelles, that is, corresponding to CMC.
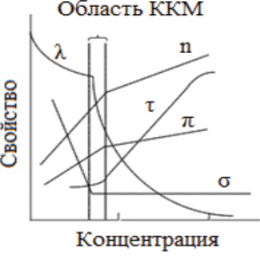
Rice. 5.3. Dependence of physicochemical quantities on surfactant concentration
The surface activity of colloidal surfactants can be approximately estimated through CMC, for example, for nonionic ![]() (for io
(for io
genic, dissociation must be taken into account).
For colloidal surfactants, the limiting values of st0 – for border
section water – air differ little and are about 71.5 – 28 = = 43.5 mJ / m 2. The CMC values differ by 2–3 orders of magnitude; therefore, it can be assumed that the surface activity of colloidal surfactants is inversely proportional to the CMC.
True solubility, that is, the equilibrium concentration of a substance that is in an aqueous solution in a molecular (or ionic) form, for such surfactants is small and amounts to hundredths or thousandths of kmol / m 3 for ionic surfactants, for nonionic surfactants it can be another one or two orders of magnitude below. Usually the true solubility of H1AB is in the range of concentrations below IG 6 -—10 3 M. The range of CMC values is in the same range.
At concentrations of surfactants in an aqueous solution that are somewhat higher than the CMC, spherical aggregates—Gartley micelles—are formed. The inner part of Gartley micelles consists of intertwining hydrocarbon radicals, the polar groups of surfactant molecules are turned into an aqueous medium. The diameter of such micelles is equal to twice the length of surfactant molecules. The number of molecules in a micelle grows rapidly within a narrow range of concentrations; with a further increase in concentration, it practically does not change (the number of micelles increases).
Spherical micelles can contain from 20 to 100 molecules or more. The number of molecules in a micelle is called the aggregation number: t= In addition to the number of micelle aggregation, the micellar mass is characterized by the micellar mass of molecules forming the unit.
Knowledge is known RTLNKKM = A ± BN, In which the character of the polar group of the Pav molecule is taken into account, in different ways manifested with micelle formation in water and non-aqueous media. In the given equation a – constant characterizing the energy dissolution of functional groups (polar parts of the molecule); B. – constant characterizing the dissolution energy per group -CH2; P – number of groups -CN2. The plus sign is used for organic solvents, "minus" – for inorganic solvents.
The introduction of electrolytes into the aqueous solutions of non-ionic surfactants poorly affects the CCM and the dimensions of the micelles; For ionic surfactants, the Ego influence substantially. The introduction of non-electrolytees (organic solvents) into the aqueous solutions of the PAV leads to a change in the CCM.
According to the results of the definition of KKM, it is possible to calculate the values of the Energy of the Association E ^ Peat molecules in a micelle by equation ![]()
where to" – Boltzmann's constant; T – absolute temperature; SO – but-